GAMP®5, 2nd Edition and Alignment with FDA’s Draft Guidance for Computer Software Assurance (CSA)
Speaker: Carolyn Troiano
Speaker Designation: FDA Compliance Consultant
Speaker: Carolyn Troiano
Speaker Designation: FDA Compliance Consultant

Technology breakthroughs have driven companies to rethink their business strategies. Once organized and regulated, these companies are now more complex, serving patients and consumers with higher expectations. To address these challenges, work habits and equipment must be updated.
Adapting to these changes requires careful attention to software development, validation, and maintaining systems throughout their life cycle. This webinar will compare the waterfall and agile approaches, exploring their pros and cons. Since no single solution fits all, understanding key factors is crucial for making informed decisions.
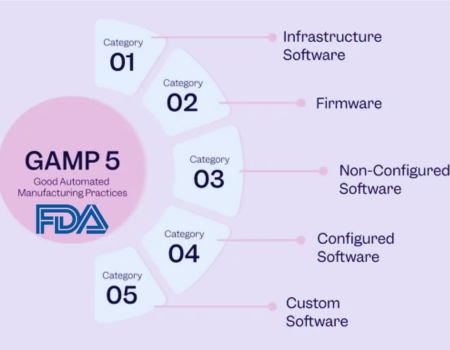
We'll map both waterfall and agile approaches to the GAMP®5 "V" Model, outlining stages for successful system validation in each. GAMP®5 (Second Edition, July 2022) aligns well with FDA Draft Guidance on Computer Software Assurance (CSA, September 2022). We'll compare CSV and CSA, emphasizing GAMP®5 best practices in validation.
The webinar will cover cloud models (IaaS, PaaS, SaaS, COTS), along with their pros and cons, best practices for meeting FDA's 21 CFR Part 11 criteria and maintaining data integrity. We'll also discuss the FDA's recent focus on modern technology, noting its shift from compliance to quality to support innovation.
The CSA guidance, issued in September 2022, promotes a risk-based, quality-centric approach over the traditional checklist-heavy CSV method. CSA encourages critical thinking, product knowledge, and quality risk management, allowing automated testing for efficient validation. FDA highlights that “WHAT” requirements can be achieved through various “HOW” methods, reducing reliance on exhaustive documentation.
For the creation of custom applications, GAMP®5 facilitates the use of incremental, iterative, and evolutionary methodologies, including automated and agile testing. Keys to success include a robust Quality Management System (QMS) and well-trained and highly disciplined teams following well-defined processes supported by tools and automation.
The participant will gain knowledge about the FDA's strategy for updating technology and how it will help the business community as well as the Agency. We will talk about how to use automated testing tools to create a continuous validation of software products, modernizing the System Development Life Cycle (SDLC) approach to Computer System Validation (CSV). The agile software development technique can be applied to this approach and modified for validation purposes. We'll go over the benefits and drawbacks of each strategy as well as successful industry best practices.
Cloud services, Infrastructure-as-a-Service (IaaS), Platform-as-a-Service (PaaS), Software-as-a-Service (SaaS), and Computer-off-the-Shelf (COTS) software will all be covered. You will get knowledge on how to choose the best possible solution and make sure that, whatever it may be, you can create a contract and Service Level Agreement (SLA) that are tailored to your needs and environment.
Rapid technological advances are driving companies to rethink strategies to manage complexity and meet rising consumer expectations. This shift demands updated work practices and tools to maintain reliable, validated systems. With the FDA’s Case for Quality initiative prioritizing quality over mere compliance, companies are encouraged to adopt automation and digital solutions for improved quality and process control. A strong Quality Management System (QMS) and clear, automated processes are essential for navigating these regulatory and technological changes successfully.
This webinar will be available soon. Please contact customer care for new schedule date.

Carolyn Troiano has more than 40 years of experience in computer systems and data in the pharmaceutical, medical device, tobacco, cannabis, and other FDA-regulated industries, as well as in banking, insurance, and government agencies. She is currently an independent consultant, advising companies on data integrity, privacy, and compliance, including implementing large-scale, complex systems, such as Enterprise Resource Planning (ERP), Customer Relationship Management (CRM), Clinical Trial Master File (TMF and eTMF), Manufacturing, Quality, and Enterprise Content Management (ECM) systems.